close
Choose Your Site
Global
Social Media


Views: 0 Author: Site Editor Publish Time: 2024-07-02 Origin: Site










The core of green hydrogen production lies in the application of efficient water electrolysis technology to produce hydrogen. Under the action of direct current, the water will undergo an electrochemical reaction and produce hydrogen and oxygen respectively in the cathode and anode of the electrolytic cell. According to the different working principles and electrolytes, water electrolysis hydrogen production technology can be divided into four types, namely alkaline water electrolysis technology (ALK), proton exchange membrane electrolysis technology (PEM), high temperature solid oxide electrolysis technology (SOEC) and solid polymer anion-exchange membrane electrolysis technology (AEM).
Alkaline water electrolysis technology (ALK) : Potassium hydroxide (KOH) solution is usually used as the electrolyte, porous membrane is used as the diaphragm, and non-precious metal nickel-based catalyst is used. The biggest advantage of this technology is mature technology, low price, as the main water electrolysis technology, the disadvantage is that the working current is relatively small, the equipment volume is large.
Proton exchange membrane electrolysis of water (PEM) : This technology replaces the diaphragm and electrolyte of alkaline electrolytic water with a proton exchange membrane, while acting as a barrier between gas and ion conduction. Among them, the proton exchange membrane thickness is thin, the resistance is small, can achieve higher efficiency and withstand larger current, the equipment volume and floor area is smaller than the alkaline electrolytic cell equipment, the operation is more flexible, the current disadvantage is that the disadvantage is the need to use expensive catalysts and fluorinated film materials, resulting in higher investment costs, and the PEM electrolytic water system structure is complex. The overall technology is now basically mature and is being commercialized.
High temperature solid oxide electrolysis water technology (SOEC) : It is a high temperature electrolysis water technology with an operating temperature of 700-1000℃. Its structure is composed of hydrogen electrode, oxygen electrode and a dense solid electrolyte (including solid zirconia, etc.). Due to the high operating temperature, it greatly increases the reaction power and reduces the power consumption, and can achieve high electrolytic efficiency, but the disadvantage is that it needs to provide a high temperature heat source.
Anion exchange membrane water electrolysis (AEM) is a new water electrolysis technology that combines the low cost advantages of alkaline electrolyzers with the high efficiency advantages of PEM. At present, AEM membrane still has mechanical and chemical stability problems, and there may be low ion conductivity, slow catalysis and other problems, and the overall technology is still in the development and demonstration stage.
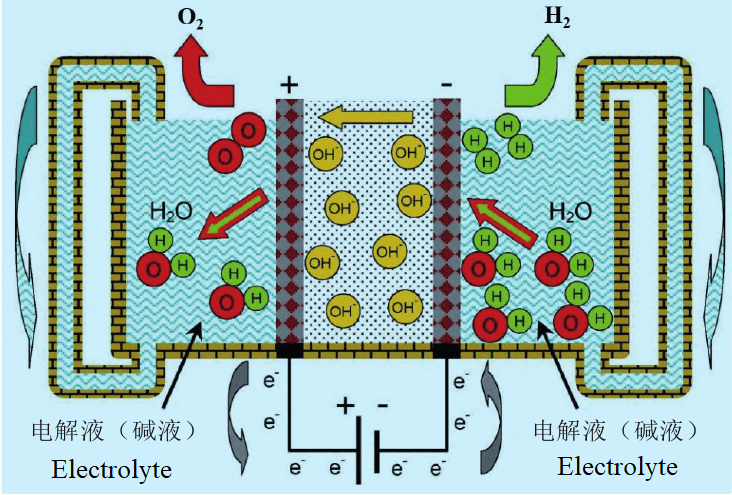
Technical principle of hydrogen production from alkaline water electrolysis
In terms of the overall structure of the electrolytic cell, the alkaline electrolytic cell is mainly composed of plate (bipolar plate + electrode frame), catalytic electrode, diaphragm, gasket and other parts. In terms of catalytic electrodes, most of the electrodes used in alkaline electrolytic cells are nickel-based materials, mostly pure nickel mesh and foam nickel as the base material, and the catalyst is coated with spray, roll coating, electroless plating and other processes to improve the electrolytic efficiency, and the catalyst is mostly nickel-based catalyst or precious metal catalyst represented by Reney nickel. In terms of diaphragm, asbestos diaphragm materials were mainly used in the early days, but the swelling of asbestos in alkaline electrolyte and the harm of asbestos to the human body made it gradually eliminated. At present, the diaphragm widely used in the industry is a new composite diaphragm based on polyphenylene sulfide (PPS). PPS as the substrate can provide a certain physical support, and PPS fabric has excellent heat resistance, high mechanical strength, excellent electrical properties. However, at the same time, the hydrophilicity of PPS material is weak, which will cause excessive internal resistance of electrolytic cell, so PPS is currently modified, such as coating polymer and zirconia to form a composite diaphragm to enhance its hydrophilicity.
In terms of plate, the plate is the support component of the alkaline electrolyzer, and its role is to support the electrode and diaphragm and conduct electricity. The domestic plate material is generally cast iron metal plate, nickel plate or stainless steel metal plate, processing method is machined into mastoid structure, and electrode frame welding after nickel plating, which nickel material is not easy to be corroded in lye, mastoid structure has support and power transmission. In terms of working principle, the alkaline electrolyzer usually uses 30% concentration of potassium hydroxide solution (KOH) or 25% concentration of sodium hydroxide solution (NaOH) as the electrolyte. Under the action of direct current, water molecules undergo hydrogen reduction reaction in the cathode to generate hydrogen and hydroxide ions. The hydroxide ions pass through the diaphragm material under the action of electric field to reach the anode. And lose electrons to form oxygen and water.
The hydrogen production system of electrolytic water mainly includes the main body of the electrolyzer and the auxiliary BOP system. The BOP auxiliary system consists of power supply equipment (power supply, transformer, rectifier, etc.), gas-liquid separation & drying and purification equipment and other equipment. Generally, the cost composition of the alkaline water electrolysis hydrogen production system is: electrolytic cell (50%), electrical equipment (15%), gas separation and drying purification equipment (15%), and other equipment (20%). In the electrolytic cell, according to IRENA data, the diaphragm and electrode assembly (57%), the pile assembly & end plate (10%), the bipolar plate (7%), the component (4%), the structural layer (14%), and the porous transmission layer (8%) are the main components of the electrolysis cost.
 |
| Working principle of ALK electrolyzer |
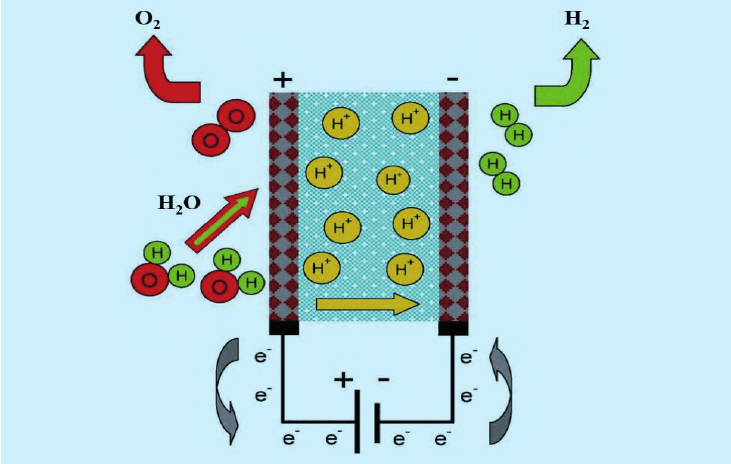
Technical principle of hydrogen production by PEM electrolytic water
PEM electrolytic cell is mainly composed of membrane electrode (including proton exchange membrane, catalyst, gas diffusion layer), bipolar plate, epoxy resin plate and end plate. Membrane electrode is the main site of material transfer and electrochemical reaction in the whole water electrolyzer, and its characteristics and structure directly affect the performance and life of PEM water electrolyzer. Proton exchange membrane: Proton exchange membrane needs to have high proton conductivity, high air tightness, high hydrophilicity, acid resistance, very low electron conductivity and other characteristics, its quality directly affects the operating efficiency and service life of the electrolytic cell. At present, most proton exchange membranes are perfluorinated sulfonic acid (PFSA) based polymers.
Catalyst: The ideal catalyst has the characteristics of corrosion resistance, good specific surface area, porosity, catalytic activity, electronic conductivity, electrochemical stability, low cost and environmental friendliness. The materials used in the anode and cathode of PEM electrolyzer are quite different, among which the cathode catalyst is mainly platinum precious metal and its alloy; The anode catalyst uses a few precious metals such as iridium and ruthenium which are resistant to oxidation and corrosion or their oxides. In terms of gas diffusion layer: the cathode usually uses carbon materials, such as carbon paper, carbon cloth and carbon felt, etc., and the anode mainly uses titanium materials such as titanium mesh, titanium plate and titanium felt. Bipolar plate: mainly used to support the membrane electrode and gas diffusion layer, while confluence hydrogen and oxygen and conduction electrons, bipolar plate needs to have high mechanical stability, chemical stability and low hydrogen permeability. The material is basically titanium, and the coating containing platinum is applied.
From the perspective of the cost composition of PEM electrolytic water hydrogen production system, the unit cost of the electrolyzer is higher, and the electrolyzer/power supply equipment/purification equipment/other equipment accounts for 60%/15%/10%/15% of the total cost, respectively. In PEM electrolyzers, the membrane electrode (24%), pile assembly & end plate (3%), bipolar plate (53%), component (3%), and multi-space transport layer (17%) are the main cost components.
 |
| Working principle of PEM electrolyzer |
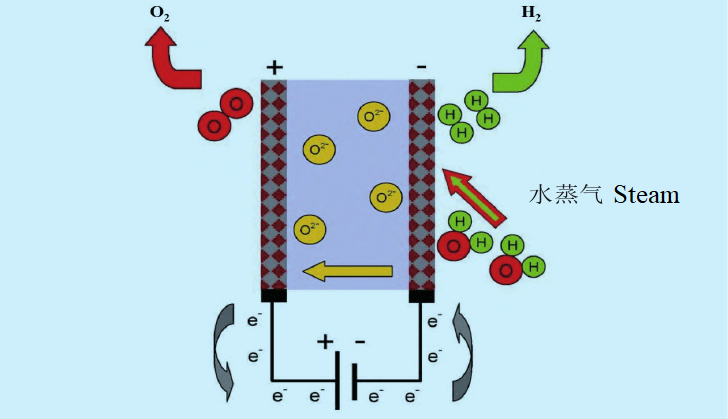
Technology principle of SOEC electrolytic water hydrogen production
SOEC (High Solid oxide electrolysis) can provide higher energy conversion efficiency than normal temperature water electrolysis technology. From the technical principle of classification, SOEC can be divided into oxygen ion conduction SOEC and proton conduction SOEC. Proton-conducting SOEC supplies high-temperature water vapor at the anode side and oxidizes, and the water molecules lose electrons to form oxygen and protons. The proton is transported through the electrolyte to the cathode and a reduction reaction occurs, where hydrogen gas is formed. Oxygen ion conduction SOEC supplies water vapor from the cathode side. Water molecules gain electrons to form hydrogen and ionize oxygen ions. Oxygen ions are carried through the electrolyte to the anode, where they are oxidized to form oxygen. At present, the commercialization of SOEC water electrolysis technology is mainly focused on oxygen ion conduction SOEC. Due to the higher requirements of proton conduction SOEC at the technical level and material selection level, the current development progress lags behind oxygen ion conduction SOEC.
The core components of SOEC electrolytic cell are electrolyte, cathode and anode, and several electrolytic cells are assembled together to form SOEC reactor. The SOEC electrolysis module is composed of multiple reactors, gas processing systems and gas conveying systems. Multiple SOEC electrolytic modules, power distribution equipment and other auxiliary equipment form a complete SOEC system. Conductive ceramic materials such as yttrium-stabilized zirconia (YSZ) and scandium-stabilized zirconia (ScSZ) are usually selected for SOEC electrolytes. The cathode needs to be in direct contact with high temperature water vapor, needs to have chemical stability at high temperature and high humidity, and needs to have similar thermal expansion properties to the electrolyte material, so the metal ceramic composite is usually used, nickel, cobalt, platinum, palladium are common SOEC cathode materials. The anode needs to be stable in a high-temperature oxidation environment, and needs to have excellent electronic conductivity, oxygen ion conductivity and catalytic activity, while the coefficient of thermal expansion also needs to match the electrolyte. At present, conductive ceramic materials prepared by perovskite oxides are the most common anode materials, among which the most representative is strontium doped lanthanum manganate (LSM).
 |
| Working principle of SOEC electrolyzer |
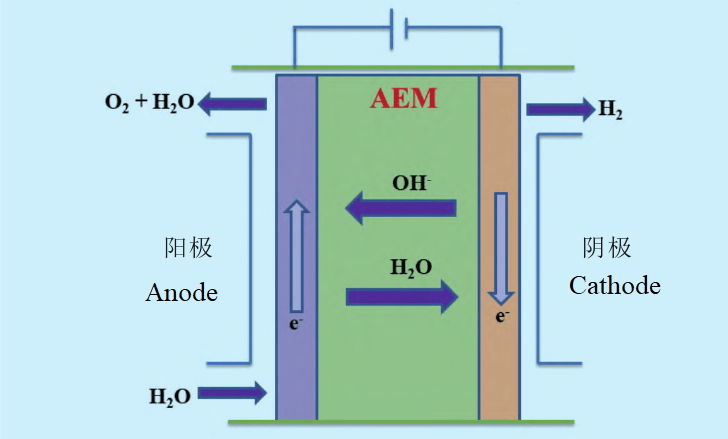
AEM electrolysis water hydrogen production technology principle
The main structure of AEM (anion exchange membrane electrolysis water technology) electrolyzer is composed of anion exchange membrane and two transition metal catalytic electrodes, generally using pure water or low concentration alkaline solution as electrolyte, and using cheap non-precious metal catalyst and hydrocarbon film. As a result, the AEM process has the advantages of low cost, quick start and stop, and low energy consumption, combining the ease of coupling with renewable energy sources while achieving the same current and efficiency as PEM. Although AEM can combine the technical advantages of PEM and ALK at the same time, it is in the initial stage of development. First of all, due to the local strong alkaline environment formed on the surface of the anion exchange membrane during the operation of AEM, the perforation caused by the degradation of AEM under the action of OH− will lead to short circuit of the reactor, which will affect the service life. Secondly, the lack of large standard products in AEM electrolyzers is also the difficulty of restricting its large-scale commercialization.
 |
| Working principle of AEM electrolyzer |